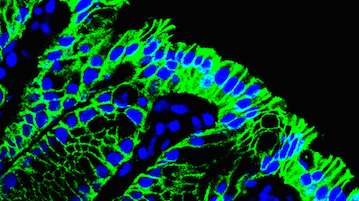
Research
Humans harbor over 100 trillion bacteria in the distal intestine. These commensal (meaning ‘share a dinning table’ in Latin) bacteria have long been appreciated for the benefits they provide to the host, the most obvious being their capacity to metabolize indigestible food components to small metabolites that are utilized as nutrients by host cells. Moreover, it is now clear that the presence of commensal bacteria contributes to shape the gut immune system though promoting the development of gut-associated lymphoid tissues (GALTs), the largest collection of secondary lymphoid organs, which are necessary for induction of mucosal IgA responses. To evoke the mucosal immune response, antigens on the mucosal surface must be transported across the epithelial barrier into GALT such as Peyer’s patches (PPs). This function, called ‘antigen transcytosis’, is mediated by specialized epithelial M cells. We are currently conducting the studies to understand the molecular machinery by which intestinal M cells facilitate antigen transcytosis.
Certain enteric bacteria also facilitate differentiation of type17 helper T (TH17) and regulatory T (Treg) cells, both of which are major T cell populations in the intestinal mucosa. In support of this concept, the development of Th17 and Treg cells as well as IgA production are defective in mice housed in a germ-free isolator (denoted as “germ-free mice”). These previous observations led to the notion that host-microbe interactions establish immunological homeostasis in the gut, which further raises the important question: how do commensal bacteria affect the host immune system? The enteric microflora constitute a potent bioreactor that controls several metabolic functions. The principal metabolic functions include the fermentation of indigestible food substances such as dietary fibers and resistant starch into simple sugars, absorbable nutrients, and short-chain fatty acids (SCFAs). Notably, certain SCFAs have been reported to exert histone deacetylase (HDAC) inhibitory activity, at least in vitro. Based on these observations, we hypothesize that intestinal microbiota may secure mucosal immune homeostasis via epigenetic mechanisms by the action of metabolites that they produce. Our laboratory aims to explore the epigenetic regulation of the immune system by intestinal microbiota, and also to elucidate the biological significance of this epigenetic regulation in gut immunity.
The specific aim of study
- Molecular basis of antigen-transcytosis by intestinal M cells
- Epithelial barrier dysfunctions and chronic inflammation
- Identification of missing link between commensal bacteria and the development of gut immune system.
References
- Hase, K. et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 462, 226–230 (2009).
- Hase, K. et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 11, 1427–1432 (2009).
- Takahashi, D. et al. The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology 141, 621–632 (2011).
- Obata, Y. et al. Epithelial cell-intrinsic notch signaling plays an essential role in the maintenance of gut immune homeostasis. J. Immunol. 188, 2427–2436 (2012).
- Kanaya, T. et al. The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nat. Immunol. 13, 729–736 (2012).
- Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011).