| ON THE COVER | 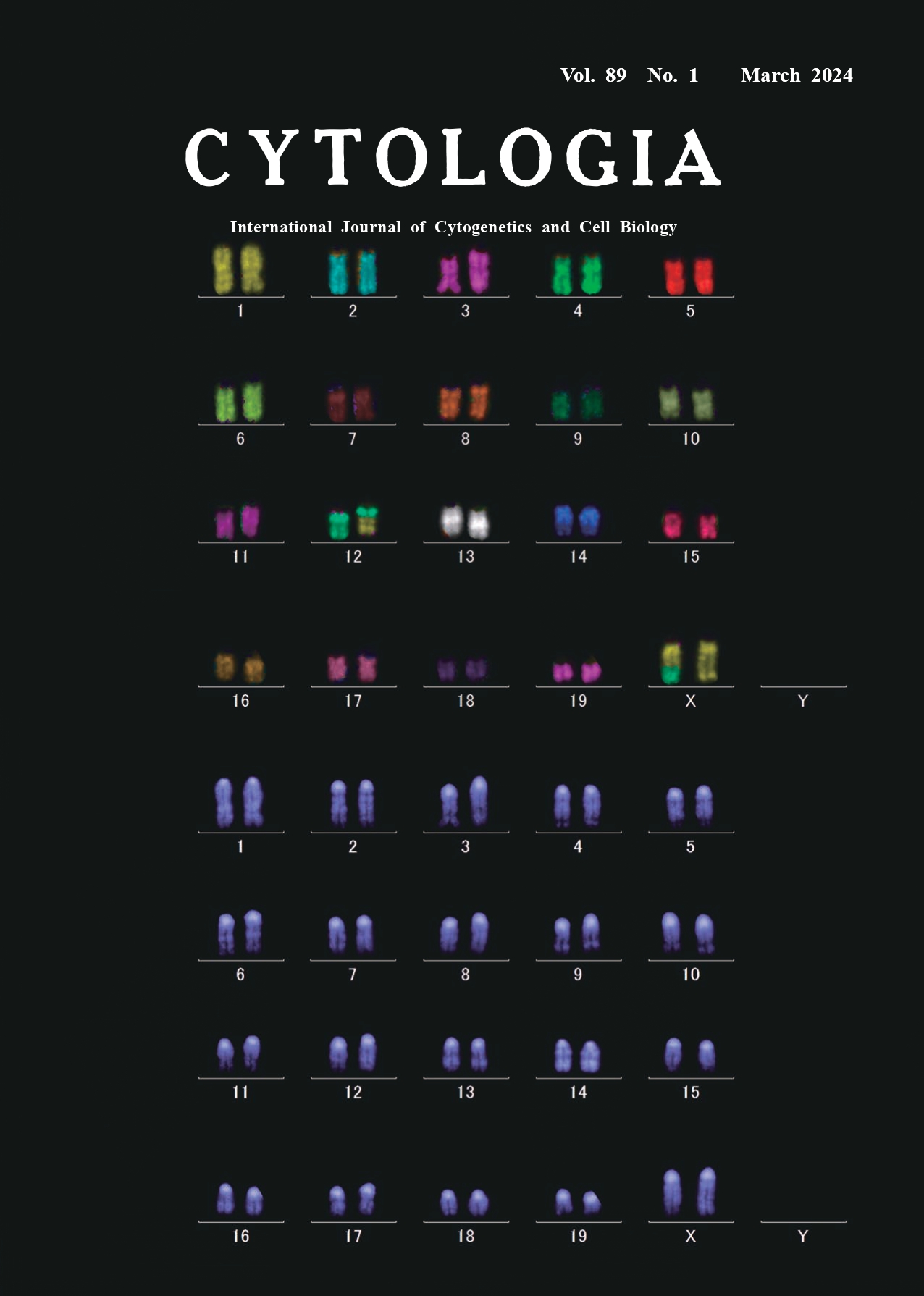 |
||
|---|---|---|---|
| Vol. 89 No.1 March 2024 | |||
| Technical Note | |||
|
|
|||
M-FISH as a sensitive tool for detecting chromosome aberrations induced by low levels of radiation Atsushi Kohda1*, Takuo Toyokawa2, Tomoyuki Umino2, and Jun-ichiro Komura1 1 Department of Radiobiology, Institute for Environmental Sciences (IES), 2–121 Hacchazawa, Takahoko, Rokkasho, Kamikita, Aomori 039–3213, Japan 2 Tohoku Nuclear Co., Ltd., 2–41–14 Higashi Okamisawa, Misawa, Aomori 033–0024, Japan
Chromosomal aberrations are produced when DNA double-strand breaks are induced in living cells by exposure to ionizing radiation. Some of these chromosomal aberrations can be detected as the changes in chromosome shape or banding patterns and are considered as one of the most sensitive and reliable indicators of the radiation exposure and especially suitable for analyzing the effects of low-dose or low-dose-rate radiation exposures, complementing/ supporting epidemiological studies. The introduction of fluorescence in situ hybridization (FISH), for staining specific chromosomal regions, increased the sensitivity and reliability of chromosome assays by detecting translocations and other chromosomal changes that were difficult to identify. Further application of multicolor fluorescence in situ hybridization (M-FISH), using probes labeled with multiple fluorophores for identifying all chromosomes at once (24 in humans, 21 in mice, 22 in rats, and 12 in Chinese hamsters), makes it possible to detect complex and fine chromosomal rearrangements. M-FISH is particularly useful for karyotyping analyses of cancer cells and genetic diseases. We have introduced M-FISH to detect chromosomal aberrations as a sensitive analytical tool to study the biological effects of low-dose or low-dose-rate radiation. The cover figure shows an M-FISH karyotyping image depicting a reciprocal translocation event between chromosomes 12 and X in a lymphocyte from a 8-weekold female C3H mouse exposed to whole body gamma ray irradiation of 500 mGy (dose rate: 700 mGy min-1), stained with the 21XMouse Multicolor FISH Probe (MetaSystems). The chromosome specimens were prepared from lymphocytes harvested from the mouse spleen and cultured for 40 h. The probe contained DNA from individual chromosomes labeled with various combinations of FITC, Orange, TexasRed, Aqua and Cy5 fluorochromes. Chromosome specimens were denatured with 0.07 N NaOH for 1 min, and DNA probes were denatured at 75°C for 5 min and hybridized at 37°C for 2 days in dark. Then, the slide was washed, air-dried, and counterstained with 4,6-diamidine-2-phenylindole (DAPI). A motorized epifluorescence microscope (Carl Zeiss, AXIO Imager Z2) and a monochrome CCD camera (Metasystems, Cool Cube 1m) was used for acquisition of images of the six different fluorochromes. The microscope and camera were controlled by the Metafer software (Metasystems). M-FISH image processing was performed by use of the ISIS software (Metasystems). The top image shows individual chromosomes distinguished by painting with different virtual colors according to the combination of the above five fluorochromes. The bottom image shows the same chromosomes stained with DAPI for identifying the location of centromeres. We analyzed approximately ~3,000 metaphases from each mouse exposed to low-dose or low-dose-rate radiation for detecting rather infrequent translocations and dicentric chromosomes. Using this method, we have reported that splenic lymphocytes from mice chronically exposed to lowdose- rates of 20 mGy day-1 and 1 mGy day-1 show a linear dose-dependent increase in translocations and dicentric chromosomes, whereas those from mice chronically exposed to a very low-dose-rate of 0.05 mGy day-1 (18.25 mGy year-1) do not show such increases (Kohda et al. 2022).
Kohda, A., Toyokawa, T., Umino, T., Ayabe, Y., Tanaka, I. B., and Komura, J. 2022. Frequencies of chromosome aberrations are lower in splenic lymphocytes from mice continuously exposed to very low-dose-rate gamma rays compared with non-irradiated control mice. Radiat. Res. 196: 639–645. * Corresponding author, e-mail: kohda@ies.or.jp DOI: 10.1508/cytologia.89.1 |
|||