| ON THE COVER | 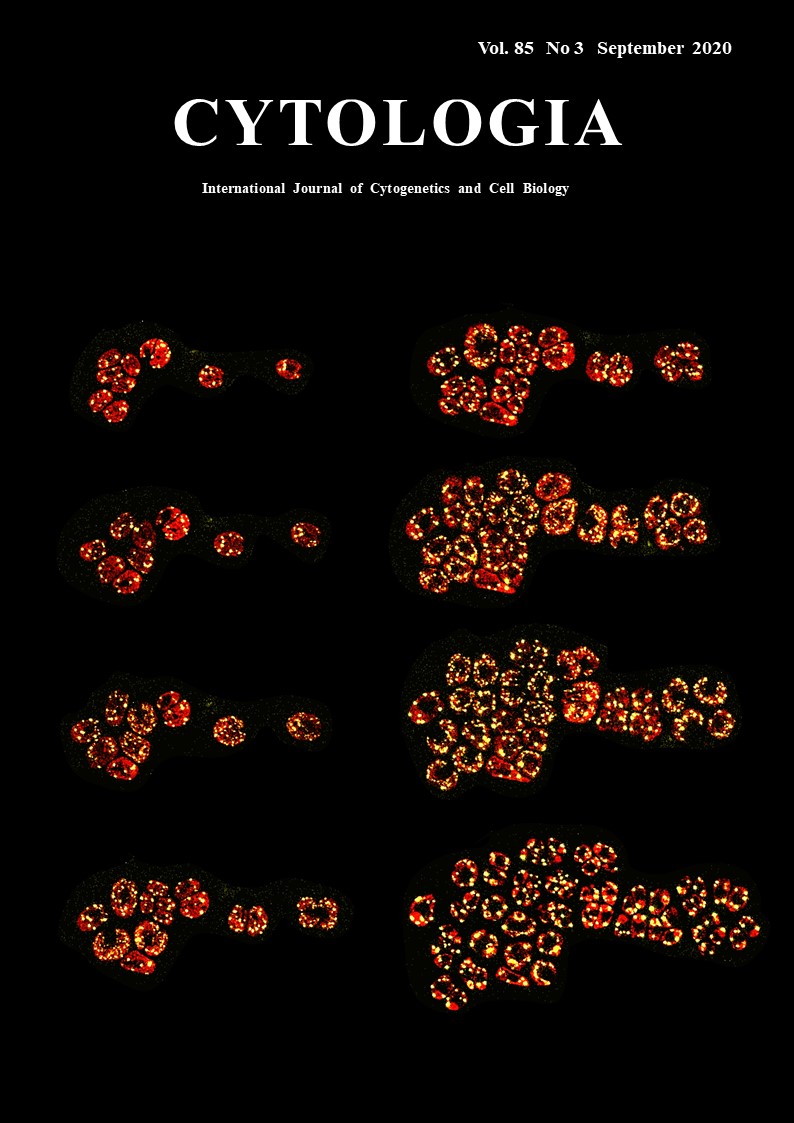 |
||
|---|---|---|---|
| Vol. 85 No.3 September 2020 | |||
| Technical Note | |||
|
|
|||
Dynamic Motion of Chloroplast Nucleoids Captured by the Microfluidic System Yoshitaka Kamimura1, Yusuke Kobayashi2 and Yoshiki Nishimura1* 1 Laboratory of Plant Molecular Genetics, Department of Botany, Kyoto University, Oiwake-cho, Kita-Shirakawa, Kyoto 606–8502, Japan 2 Graduate School of Science and Engineering, Department of Science, Ibaraki University, Ibaraki 310–8512, Japan
Chloroplasts generally possess multiple copies of chloroplast (cp) genomes which are organized into cpDNA-protein complexes, called cp nucleoids. Cp nucleoids change drastically during the cp divisions, differentiation, life cycle and evolution. In this research, the dynamic behaviors of cp nucleoids were captured by the live-imaging system based on the microfluidic technology (Kamimura et al. 2018). The unicellular green alga Chlamydomonas reinhardtii is an ideal model organism for the analysis of cp nucleoids because each of cells harbors just one chloroplast and the divisions can be readily synchronized by controlling the light/dark condition. In C. reinhardtii, ~80 cpDNA copies are organized into 5–10 cp nucleoids (~1.5 μm) that can be clearly observed by fluorescence microscopy. Although SYBR Green I is a commonly used fluorochrome for visualizing DNA molecules in living cells, it is unstable and unsuitable for time-lapse imaging. Furthermore, in addition to cp nucleoids, SYBR Green I stains mitochondrial nucleoids and cell nuclei, making it difficult to precisely track cp nucleoid movements. Therefore, we prepared a strain expressing a yellow fluorescent protein (YFP) fused to heat unstable (HU) protein, which is one of the major cp nucleoid proteins. The fluorescence of HU:YFP was stable throughout the cell cycle. Additionally, the morphological changes to cp nucleoids were clearly visualized during cell/cp divisions. A microfluidic system [CellASIC™ ONIX Y04C-02 Microfluidic Yeast Plates with the CellASIC™ Microfluidic system (Merck, Kenilworth, NJ, USA)] was employed to trap viable cells. The microfluidic system comprised a micro-chamber and pumps. The cells were infused into the chamber where they were trapped between narrow gaps (3.5–4.5 μm). The cells were continuously provided fresh growth medium (Tris-acetate-phosphate: TAP) to maintain their viability. The microfluidic platform was placed on the stage of a confocal laser microscope SP5 (Leica Microsystems, Wetzlar, Germany) equipped with one Hybrid detector (HyD) and two photon multiplier tubes (PMTs). HyD and PMT1 was used to detect YFP signal (520–582.3 nm) and chlorophyll signal (661–721 nm), respectively. Z-stack images were obtained periodically. In the figure, serial images of C. reinhardtii cells expressing HU:YFP (yellow) captured from the video are shown. They are arranged chronologically from the left top to the right bottom. During the G1 phase, 5–10 cp nucleoids (yellow) were observed in one chloroplast. Prior to the initiation of cell/ cp division, small particles (~0.1–0.5 μm) started to detach from the cp nucleoids, which decreased in size as the number of particles increased. Additionally, the small particles moved back and forth to connect the cp nucleoids. Eventually, cp nucleoids formed a network-like structure that spread throughout the chloroplast, which divided twice to form four daughter chloroplasts after the network-like structure formed. The small particles quickly fused to form a separate globular structure after the chloroplast divided. These observations suggest that the cp nucleoid would be a transformable network consists of a highly compact core with proximal areas associated with cpDNA replication and nucleoid formation.
Kamimura, Y., Tanaka, H., Kobayashi, Y., Shikanai, T. and Nishimura, Y. 2018. Chloroplast nucleoids as a transformable network revealed by live imaging with a micro fluidic device. Commun. Biol. 1: 47. * Corresponding author, e-mail: yoshiki@pmg.bot.kyoto-u.ac.jp DOI: 10.1508/cytologia.85.177 |
|||