| ON THE COVER | 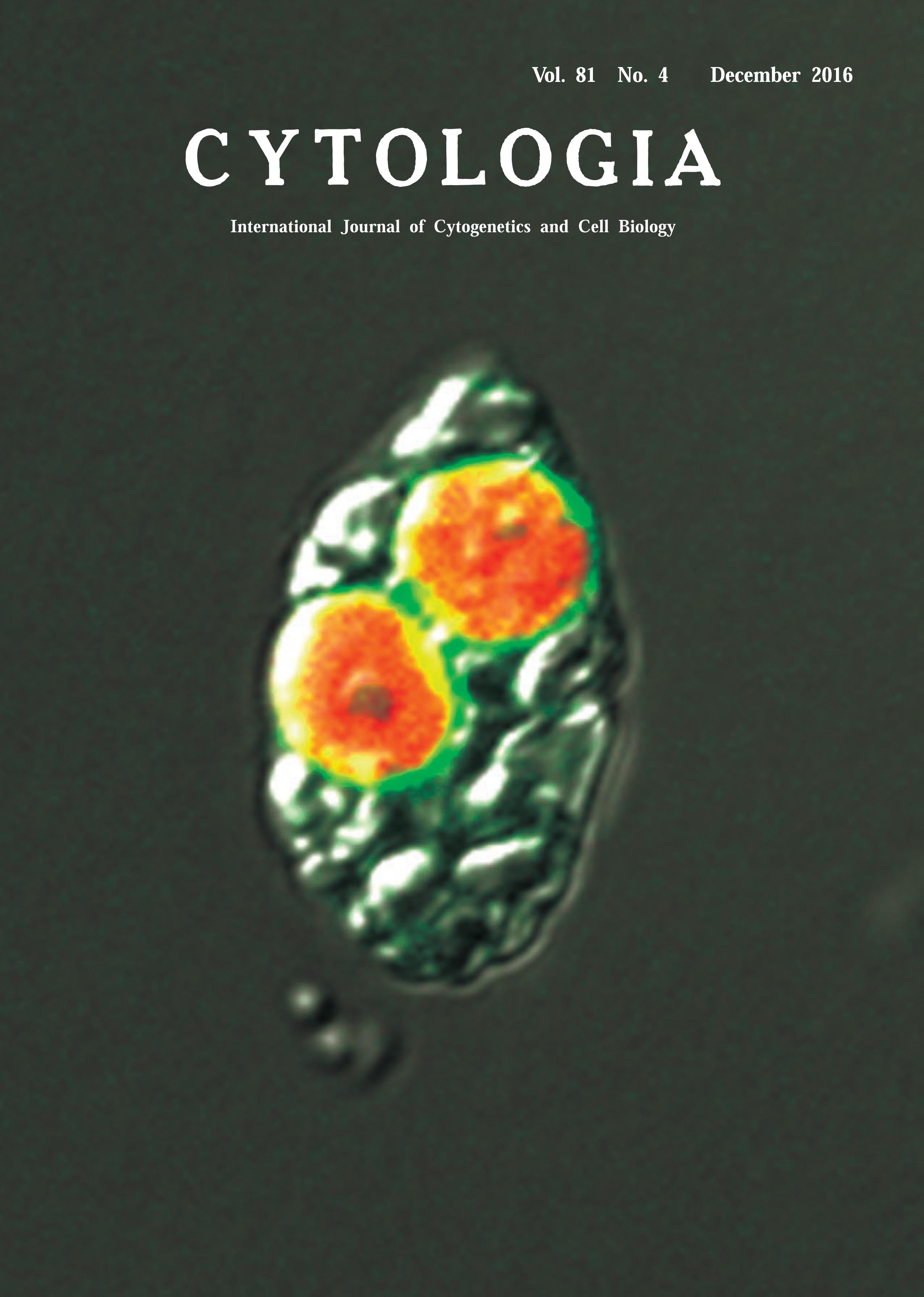 |
|
|---|---|---|
| Vol. 81 No.4 Decemer 2016 | ||
| Technical Note | ||
|
|
||
| Visualization of Cyanelle Peptidoglycan in Cyanophora paradoxa Using a
Metabolic Labeling Method with Click Chemistry Haruna Higuchi1 Katsuaki Takechi2 and Hiroyoshi Takano2,3* 1Faculty of Science, Kumamoto University, Kumamoto 860–8555, Japan 2Faculty of Advanced Science and Technology, Kumamoto University, Kumamoto 860–8555, Japan 3Institute of Pulsed Power Science, Kumamoto University, Kumamoto 860–8555, Japan Received October 12, 2016; accepted October 20, 2016 It is widely accepted that a symbiotic relationship between a cyanobacterium and a mitochondrial host led to the origin of the plastids found in primary photosynthetic eukaryotes such as glaucophytes, red algae, and green plants. Among them, glaucophytes are a small group of algae comprised of about 10 species that contain cyanelles, unique plastids with two envelopes and peptidoglycan between them. Previously, we visualized plastid peptidoglycan in moss by epifluorescence microscopy using metabolic labeling with click chemistry (Hirano et al. 2016). In this Technical Note, we applied the same method to visualize the cyanelle peptidoglycan in glaucophytes. The glaucophyte alga Cyanophora paradoxa (strain NIES-547; National Institute for Environmental Studies, Tsukuba, Japan) was cultured in C medium (see the Microbial Culture Collection at the NIES homepage: http://mcc.nies.go.jp) at 23°C under continuous light (15 μmol/m2 s). C. paradoxa cells grown for two weeks were transferred to fresh medium at a concentration of 0.43±0.03×106 cells/mL. D-Cycloserine, a D-Ala: D-Ala ligase (DDL) and alanine racemase inhibitor, was added to the culture of 1.7±0.1×106 cells/mL at a final concentration of 5 μM five days after transfer. D-Cycloserine was used as an antibiotic reagent, as the integration of D-alanyl-D-alanine (DA-DA) generated by DDL into the end of the peptide part of peptidoglycan is essential for its biosynthesis. C. paradoxa was grown to 3.7±0.2×106 cells/mL in medium without D-cycloserine until eight days after transfer, and the concentration of cells decreased to 1.2±0.1×106 cells/mL in the D-cycloserine medium. The inhibition of C. paradoxa growth by D-cycloserine was caused by interference with cyanelle division, similar to what occurs during ampicillin treatment. Growth of cells treated with D-cycloserine recovered to 2.5±0.1×106 cells/mL eight days after transfer when cells were cultured in medium with ethynyl DADA (EDA-DA), suggesting incorporation of EDA-DA into the cyanelle peptidoglycan instead of DA-DA. C. paradoxa cells grown with D-cycloserine and EDADA were washed three times with medium, fixed, and permeabilized with 2.5% formalin, 0.1 M PIPES-NaOH (pH 6.8), 2.5 mM EGTA, 1 mM MgCl2, 0.01% NP-40, 1% DMSO, and 0.5 mM PMSF for 1 h, then washed once with 1% bovine serum albumin in 1× PBS. The samples were subjected to click chemistry to attach an azidemodified Alexa 488 fluorophore to the EDA-DA probe with a Click-iT Cell Reaction Buffer Kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Carlsbad, CA, U.S.A.). Confocal laser scanning microscopic images were captured using a Fluoview FV1200 (Olympus, Tokyo, Japan). The Alexa 488 and chlorophyll fluorescence images were merged into an 11.8-μm wide bright-field image of a C. paradoxa cell. No fluorescence was detected in C. paradoxa cells grown in medium containing D-cycloserine and DA-DA (data not shown). This result shows that the cyanelle peptidoglycan can be detected by EDA-DA metabolic labeling with click chemistry.
Hirano, T., Tanidokoro, K., Shimizu, Y., Kawarabayasi, Y., Ohshima, T., Sato, M., Tadano, S., Ishikawa, H., Takio, S., Takechi, K. and Takano, H. 2016. Moss chloroplasts are surrounded by a peptidoglycan wall containing D-amino acids. Plant Cell 28: 1521–1532. *Corresponding author, e-mail: takano@kumamoto-u.ac.jp DOI: 10.1508/cytologia.81.357 |
||