| ON THE COVER | 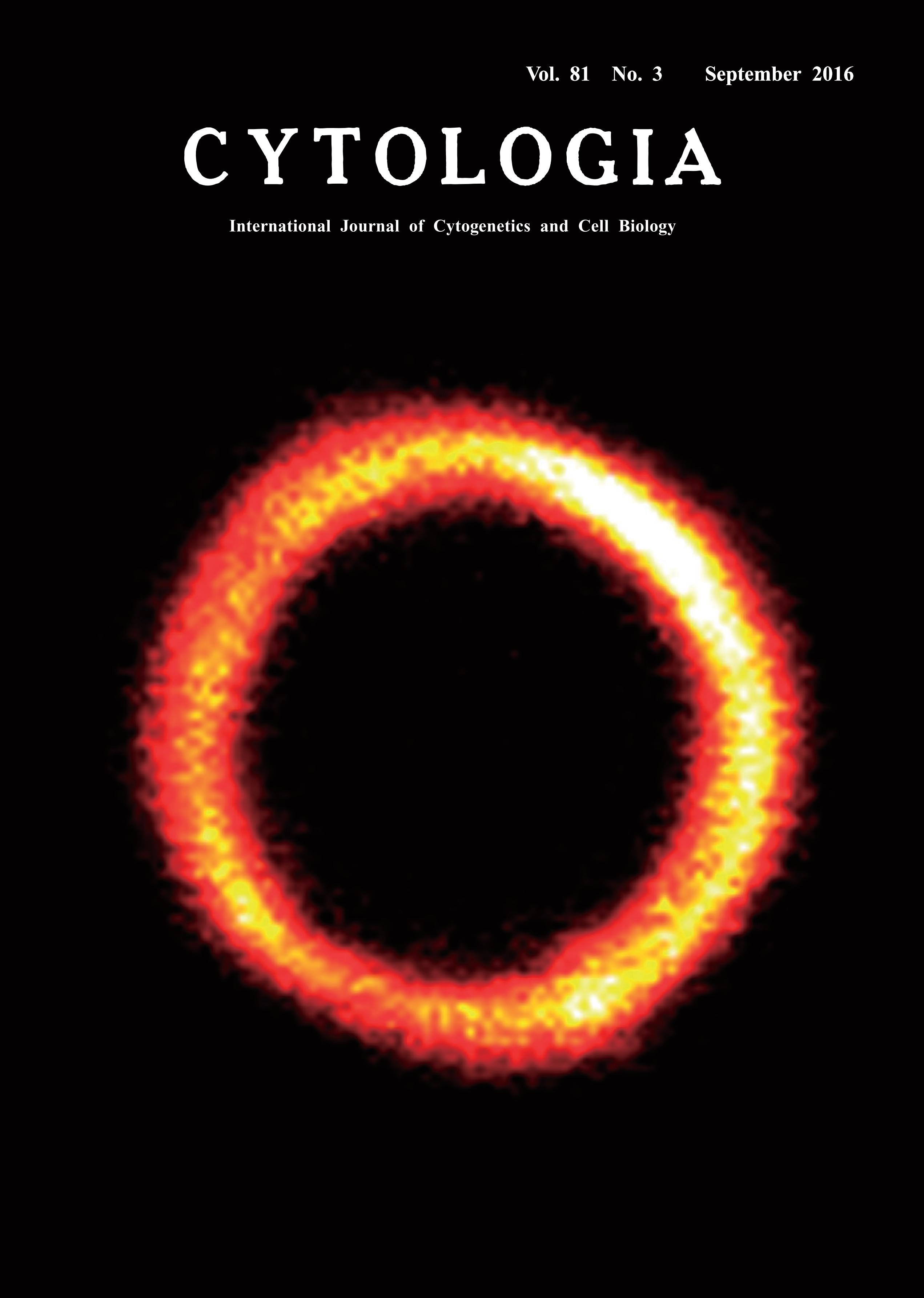 |
|
|---|---|---|
| Vol. 81 No.3 September 2016 | ||
| Technical Note | ||
|
|
||
| Reconstitution of the Chloroplast FtsZ Ring Using the Heterologous Yeast
System Pichia pastoris Yamato Yoshida1* and Yuko Mogi2 1 Laboratory for Single Cell Gene Dynamics, Quantitative Biology Center, RIKEN, Furuedai, Suita, Osaka 565–0874, Japan 2 Department of Biological Sciences, Graduate School of Science, University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113–0033, Japan Received July 9, 2016; accepted July 11, 2016 Chloroplasts (plastids) increase multiplicatively via division, which is mediated by a duplex ring complex called the plastid-dividing (PD) machinery (Yoshida et al. 2012). The PD machinery includes inner and outer ring structures that assemble at the chloroplast division site and cleave the chloroplast into two daughter chloroplasts. During the chloroplast division, the FtsZ ring assembles on the stromal side of the chloroplast inner envelope membrane as the inner ring of the PD machinery (Yoshida et al. 2008). Chloroplast FtsZs are tubulin-like GTPases that descended from a precursor in the cyanobacterial ancestor of chloroplasts. In bacteria, FtsZ proteins assemble into a single ring beneath the cell membrane and provide the contractile force to drive bacterial cell division. Interestingly, whereas bacteria have one FtsZ gene in their genomes, the chloroplast FtsZ gene was duplicated and these duplicated loci, now present in the nuclear genomes, are widely conserved throughout photosynthetic eukaryotes. However, the mechanisms of FtsZ-ring assembly and constriction in the PD machinery and the functional significance of the duplication and retention of two FtsZs (FtsZ1 and FtsZ2) during chloroplast evolution are not fully understood. Recently, we demonstrated that chloroplast FtsZs can spontaneously assemble into single rings that can constrict in the absence of any other chloroplast division proteins by using the heterologous yeast system Pichia pastoris. Our findings also provided evidence that FtsZ2 and FtsZ1 form heteropolymers, suggesting that protofilaments of chloroplast FtsZs are structurally similar to those formed by α-tubulin and β-tubulin (Yoshida et al. 2016). The GenBank accession numbers for the FtsZ genes are as follows: cDNAs of Arabidopsis thaliana FtsZ2 (AF089738) and A. thaliana FtsZ1 (AY113896). The X-33 strain of P. pastoris was used in this study. Transformation was performed with the pPICZ A expression vector (Invitrogen). Fluorescence images were captured using a laser-scanning confocal microscope FluoView 1000 (Olympus). Co-expressed FtsZ1 and FtsZ2 scattered and were likely to co-localize in the cytosol from 2 to 4 h after moderate induction. Then, a short filament accumulated from punctate units and grew into a single and uniformed ring at 8 h in the yeast cell. In these cells, a high concentration of FtsZ protein in the ring activates its constriction.
Yoshida, Y., Mogi, Y., TerBush, A. D. and Osteryoung, K. W. 2016. Chloroplast FtsZ assembles into a contractible ring via tubulinlike heteropolymerization. Nat. Plants 2: 16095. Yoshida, Y., Nishida, K., Kuroiwa, T. and Kawano, S. 2008. Novel dynamics of FtsZ ring before plastid abscission. Cytologia 73: 197–201. *Corresponding author, e-mail: yamato.yoshida@riken.jp DOI: 10.1508/cytologia.81.249 |
||