| ON THE COVER | 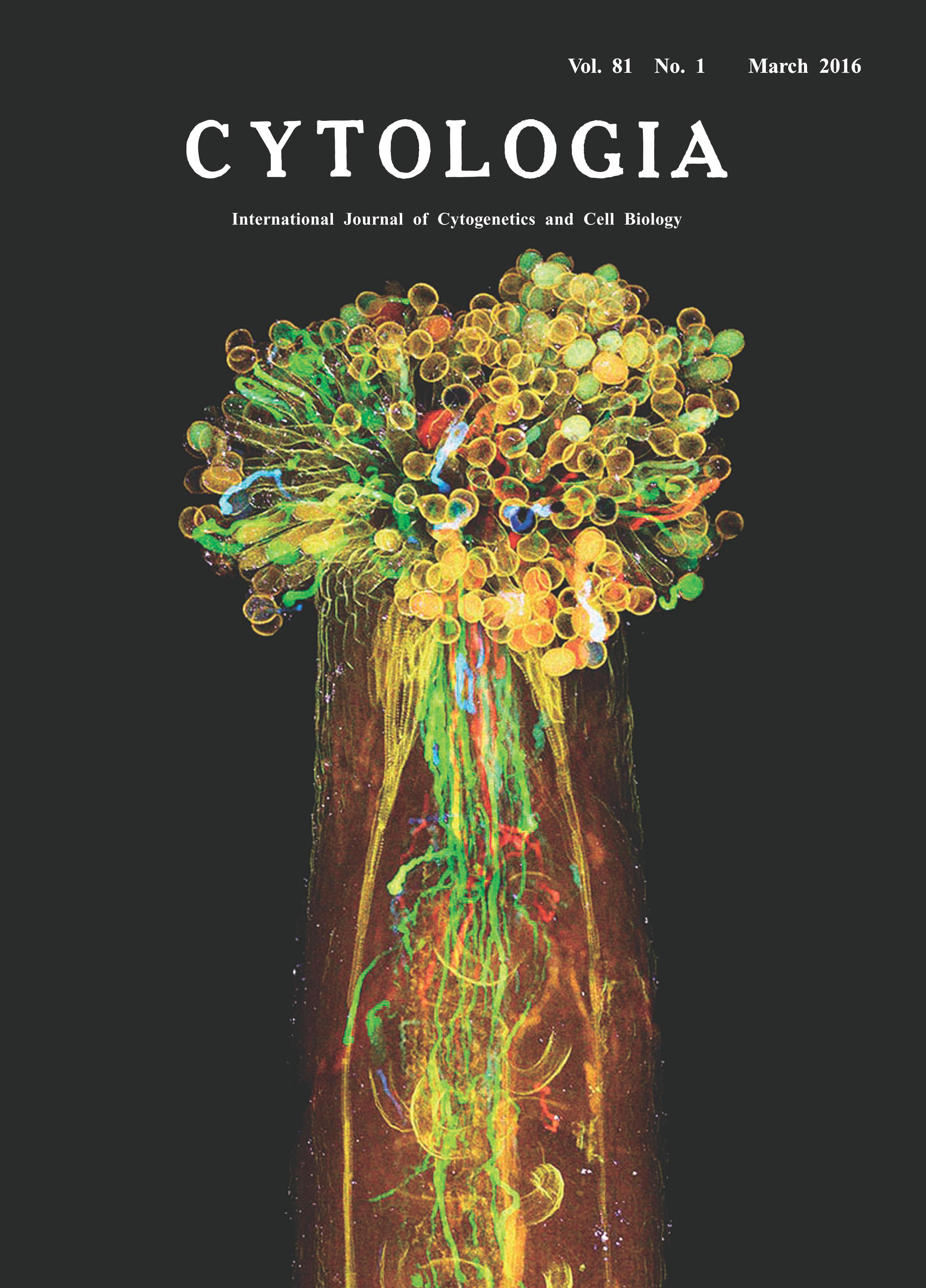 |
|
|---|---|---|
| Vol. 81 No.1 March 2016 | ||
| Technical note | ||
|
|
||
| Visualization of Plant Sexual Reproduction in
the Whole-mount Pistil by ClearSee Yoko Mizuta1,2*, Daisuke Kurihara1,2and Tetsuya Higashiyama1,2,3 1 Graduate School of Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi 464–8602, Japan 2 JST, ERATO, Higashiyama Live-Holonics Project, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi 464–8602, Japan 3 Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi 464–8602, Japan Received October 16, 2015; accepted October 17, 2015 In flowering plants, sexual reproduction begins with pollination, the transfer of pollen from anther to stigma of the pistil. The pollen tube geminated from pollen including sperm cells plays an essential role in this reproductive process, because the egg cell in the ovule is deeply embedded in the pistil. Previously, how pollen tube delivers sperm to egg cells has been analyzed by staining with aniline blue solution, but different genotypes of pollen tubes are indistinguishable with this method. Here we attempted to visualize each pollen tube inside the whole-mount pistil of the Arabidopsis thaliana by new clearing method, ClearSee (Kurihara et al. 2015). ClearSee can diminish chlorophyll autofluorescence and adjusts the refractive index mismatch while preserving the fluorescence of a variety of fluorescent proteins. Therefore, ClearSee allows deep imaging to reveal the inside structure and phenomena in plant tissues. The pistil of A. thaliana was pollinated with each pollen from LAT52pro::mTFP1, LAT52pro::sGFP, LAT52pro:: Venus, and LAT52pro::TagRFP transgenic plants, and then collected 6 h after pollination. The pollinated pistil was fixed with 4% paraformaldehyde under the vacuum for 30 min. After washing with PBS (pH 7.4) twice, the pollinated pistil was treated with ClearSee solution for 4 weeks. Fixation and clearing procedures were carried out at room temperature. Spectrum images were obtained from 118 z-stacks with 3.0-μm intervals by two-photon microscopy (A1R MP, Nikon, Tokyo, Japan) with simultaneously excitation at 980-nm (Mizuta et al. 2015). Images were processed with NIS-Elements AR software 4.10 (Nikon) to create maximum-intensity projection images and to add color. Each pollen tube from LAT52pro::mTFP1, LAT52pro:: sGFP, LAT52pro::Venus, and LAT52pro:: TagRFP was obviously distinguished, indicating that ClearSee differentiated the pollen tubes of distinct genotypes within the pistil. It is also possible that each fluorescent protein of pollen tubes was observed even after treatment with ClearSee for 5 months. This method will be useful to analyze plant sexual reproduction without any dissections.
Mizuta, Y., Kurihara, D. and Higashiyama, T. 2015. Two-photon imaging with longer wavelength excitation in intact Arabidopsis tissues. Protoplasma 252: 1231–1240. *Corresponding author, e-mail: mizuta.yoko@f.mbox.nagoya-u.ac.jp DOI: 10.1508/cytologia.81.1 |
||