| ON THE COVER | 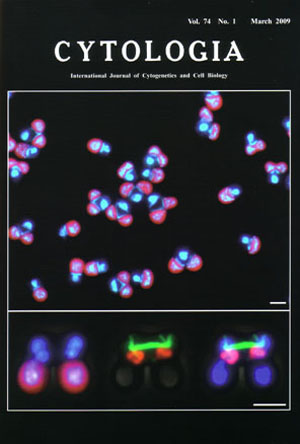 |
|---|---|
| Vol. 74 No.1 March 2009 | |
| Technical note | |
|
|
|
| Synchronization of cell nuclear, mitochondrial
and chlroplast divisions in the unicelluar red alga Cyanidioschyzon merolae The benefit of synchronous cultures is that they provide enough material for detailed observations of fine structure and bulk biochemical measurements in cells at a specific stage during the mitotic cycle. The upper image shows fluorescence micrograph of dividing cells during mitotic phase in synchronized culture. The cell nuclei, mitochondrial nuclei (nucleoids) and chloroplast nuclei (nucleoids) emit blue-whitc fluorescence after staining with 4',6-diamidino-2-phenylindole (DAPI) and chloroplasts emit red-autofluorescence (see Fujiwara et al. 2009. DNA Res. 16: 59-72). The dividing cells each contain one cell nucleus, one mitochondrial nucleus and two chloroplast nuclei. The organelle divisions promote in the order of a chloroplast, a mitochondrion and a cell nucleus. The bottom images show fluorescence/phase contrast micrographs showing a dividing cell at anaphase after triple staining with DAPI (blue-white in left and merge of right), mouse anti-tubulin antibody (green in middle and right), guinea pig anti-mitochondrial porin antibodies (orange in middle and merge). The cell nuclei (top in left and right), mitochondrial nuclei (middle in left and right) and chloroplast nuclei (bottom in left and right) are observed. When cytokinesis begins, the spindle pole bodies directly associate mitochondria and nuclear envelope (middle, right). Microtubules form a thick spindle, which pull daughter mitochondria and push daughter cell nuclei to the opposite site. Bar (upper, bottom): 2 µm. Cyanidioschyzon merolae has minimum set organelles, and the organelle division can be easily synchronized under the light/dark cycles. The genome of cell nucleus, mitochondria and plastid were completely '100%' sequenced. The omics of C. merolae greatly promotes analyses of organelle division. The synchronized cells were fixed in 1% glutaraldehyde and stained with 1µg/ml DAPI. For the immunostaining, the cells were fixed in 2% paraformaldehyde in dissolved in methanol at -20°C for 5 min. DNA was stained with DAPI. The microtubule was stained with primary antibodies against ƒ¿-tubulin/CMT504C and secondary allexa488-conjugated goat anti-mouse IgG. The mitochondria were stained with primary antibodies against mitochondrial porin/CMO111C and secondary allexa555-conjugated goat antiguinea pig. (Takayuki Fujiwara, Yamato Yoshida and Tsuneyoshi Kuroiwa, Research Information Center for Extremophile, Rikkyo University, 3-34-1 Nishiikebukuro, Toshima, Tokyo 171-8501, Japan) |
|