[-> Archives of Dr. mn's Research Works]
The 16th World Conference of Anatomic and Clinical Pathology Abstracts 10, 1991
A Total Automation System for the Clinical Laboratory
Masahiro NISHIBORI*1 and Shin-ichi SHIINA*2
*1,2Department of Laboratory Medicine, Tokyo Medical and Dental University, Tokyo, 113-8519, Japan
[abstracts and a presented poster with detailed explanation]
[Abstracts] We present details of our concept for a total automation system for use in a clinical laboratory, which is now under development with the aim of supporting medical practice in the 21st century. It is completely different from its predecessors and is summarized as follows. (1) Total investigation: Almost all of the functions required of a clinical laboratory, which include not only assessment of the degree of damage to affected organs, but also investigating the cause of diseases, using any kind of body fluid, are considered. (2) Total automation: The system's automatic function covers not only analysis, but also separation of sera from blood specimens, transfer of specimens, maintenance of instruments, supply of reagents and so on. (3) Total safety: Our system has full safeguards to avoid any possible hazards to operators and the global environment. (4) Total flexibility: Any functions of our system can be modified according to changes in the requirements of a clinical laboratory, and our system should be able to manage specimens showing various degrees of differences. (5) Total standardization: In order to make our system not only flexible, but also compatible with systems applied in other laboratories, we propose standardization of the interface between its elements. (6) Total open-door policy: Our plan is intended to be fully open to anyone who wishes to participate, and its benefits are fully extended to everyone. (7) Total estimation: In order to judge whether our project is practical, its total benefits as well as its total cost should be estimated.
|
Poster
Up to now, several clinical laboratories have made considerable efforts to become automated to some extent, using automatic analyzers, sampling machines, conveyors, factory robots and computers, and a practical breakthrough in clinical laboratory procedures has been expected for some time. However, the general implementation of such automation systems has been prevented by certain factors.
|
Seven "totals" of our system
(1) Total investigation
(2) Total automation
(3) Total safety
(4) Total flexibility
(5) Total standardization
(6) Total open-door policy
(7) Total estimation
|
In order to overcome these problems, we have started a new project for development of a total automation system for the clinical laboratory, with the aim of supporting medical practice in the 21st century. Our system is based on a completely different concept from its predecessors and includes several new ideas. These are summarized as seven so-called "totals", which will be dealt with in order.
|
(1) Total investigation
Purpose
- To measure damage to affected organs
- To investigate the cause of diseases
Function
- Routine tests
- Tests for emergency care
- Microbiological tests
- Genetic examinations
Test specimens
- Blood
- Urine
- Cerebrospinal fluid
- Sputa and other body fluids
|
The first of these is total investigation.
We considered almost all of the functions required of a clinical laboratory by a physician in order to investigate any diseases for subsequent treatment. We intended that our system should be able to not only measure damage to affected organs, but also to investigate the cause of diseases. This aspect covers routine tests, tests used in emergency care, microbiological tests and genetic examinations. The system should be able to examine any kind of body fluid expected to be a test specimen.
|
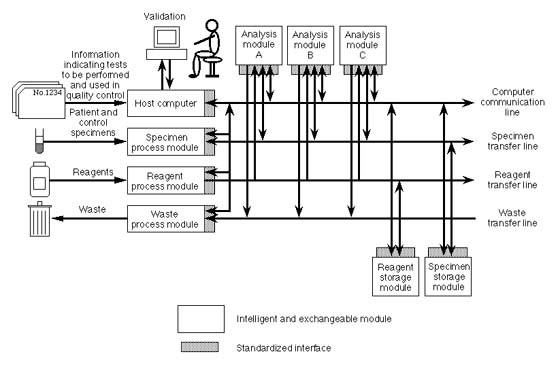
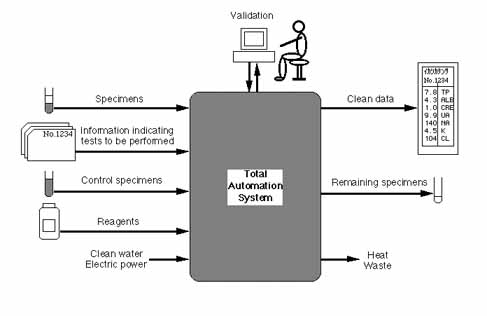
(2) Total automation
Not only:
- Analysis
But also:
- Separation of sera from blood specimens
- Transfer of specimens
- Maintenance of instruments
- Supply of reagents
- Collection and storage of remaining specimens
- Collection and processing of heat and waste
Excepting:
- Final validation of the output data
|
|
The second aspect is total automation.
Specimens are entered into our system following information indicating tests that have to be performed, and other necessary items include control specimens, reagents, clean water and a source of electric power. Clean data are obtained, leaving residual specimens, heat and waste. Therefore, the system's automatic function covers not only analysis, but also separation of sera from blood specimens, transfer of specimens, maintenance of instruments, supply of reagents, collection and storage of remaining specimens, and collection and processing of heat and waste. Of course, this will include functions for quality control, but final validation of the output data should be done by a clinical laboratory specialist.
|
(3) Total safety
Safeguards for operators
- Mechanical hazards
- Physical hazards
- Chemical hazards
- Biological hazards
Prevention of environmental pollution
- Chemical pollution
- Biological pollution
- Radioactive pollution
- Heat pollution
|
The third aspect is total safety.
Our system has full safeguards to protect operators from any possible hazards, and it can process heat and waste so as to avoid environmental pollution.
|
(4) Total flexibility
To incorporate developments in technology and medicine
To allow for differences among specimens
|
The fourth consideration is total flexibility.
To maintain consistent usefulness of our system, any of its functions can be modified according to changes in clinical laboratory requirements, which inevitably occur in parallel with developments in technology and medicine.
Furthermore, unlike an ordinary automated factory, which only deals with homogeneous products, our system should be able to manage specimens showing various degrees of differences among them, necessitating sufficient flexibility to deal with them.
|
(5) Total standardization
To maintain sufficient flexibility
To offer a standard for similar systems
|
The fifth consideration is total standardization.
In order to make our system not only flexible, but also compatible with systems applied in other places, we propose standardization of sampling cups, the interface between transfer and analysis equipment, the interface between computers and analyzers, and so on. We are developing not only a custom-built system which is most suitable for our own hospital, but also a standardized foundation on which anyone working in clinical laboratory can build a desired system, using appropriate components selected from among many standardized products supplied by various makers. Such an environment would greatly assist in the standardization of not only the values of clinical tests, but also their quality.
|
(6) Total open-door policy
Participation in development
Use of the benefits of the system
|
The sixth aspect is a total open-door policy.
Our plan is intended to be fully open to anyone who wish to participate, and its benefits are fully extended to everyone. Therefore, we wish to gather and integrate as many promising technologies as possible being developed elsewhere, and eliminate any restriction on their potential.
|
(7) Total estimation
Total cost
- Initial investment
- Running costs
Total benefit
- Direct effects
Improvement in quality of laboratory data
Improvement in use of laboratory data
Decrease in examination time
Saving of man power
- Indirect effects
Direct effects at other laboratories
Saving of manufacturing cost of products
Improvement of products through competition
|
The seventh goal is total estimation.
In order to judge whether our project is practical, its total benefits as well as its total cost should be estimated. The investment shown here, which is required to complete our concept may seem extravagant, but among the benefits are not only direct effects obtained at our laboratory, but also indirect effects of standardization, for example, direct effects on other laboratories using know-how established at our laboratory, saving of manufacturing cost for developing many versions of machines according to variable standards all of which have the same function, and improvement of products by competition among makers stimulated by fair standardization.
Last year, we sent descriptions of our project to over three hundred companies who might eventually be involved, and we received many satisfactory responses. And we have already selected one company as a total organizer of the project, which has just started.
Finally, we plan to install our system in the new building of our hospital which is presently under construction. We invite all those who are interested to visit our hospital in 4 years time, when it is hoped the system will be completed and operational.
|
[-> Archives of Dr. mn's Research Works]
|