| ON THE COVER | 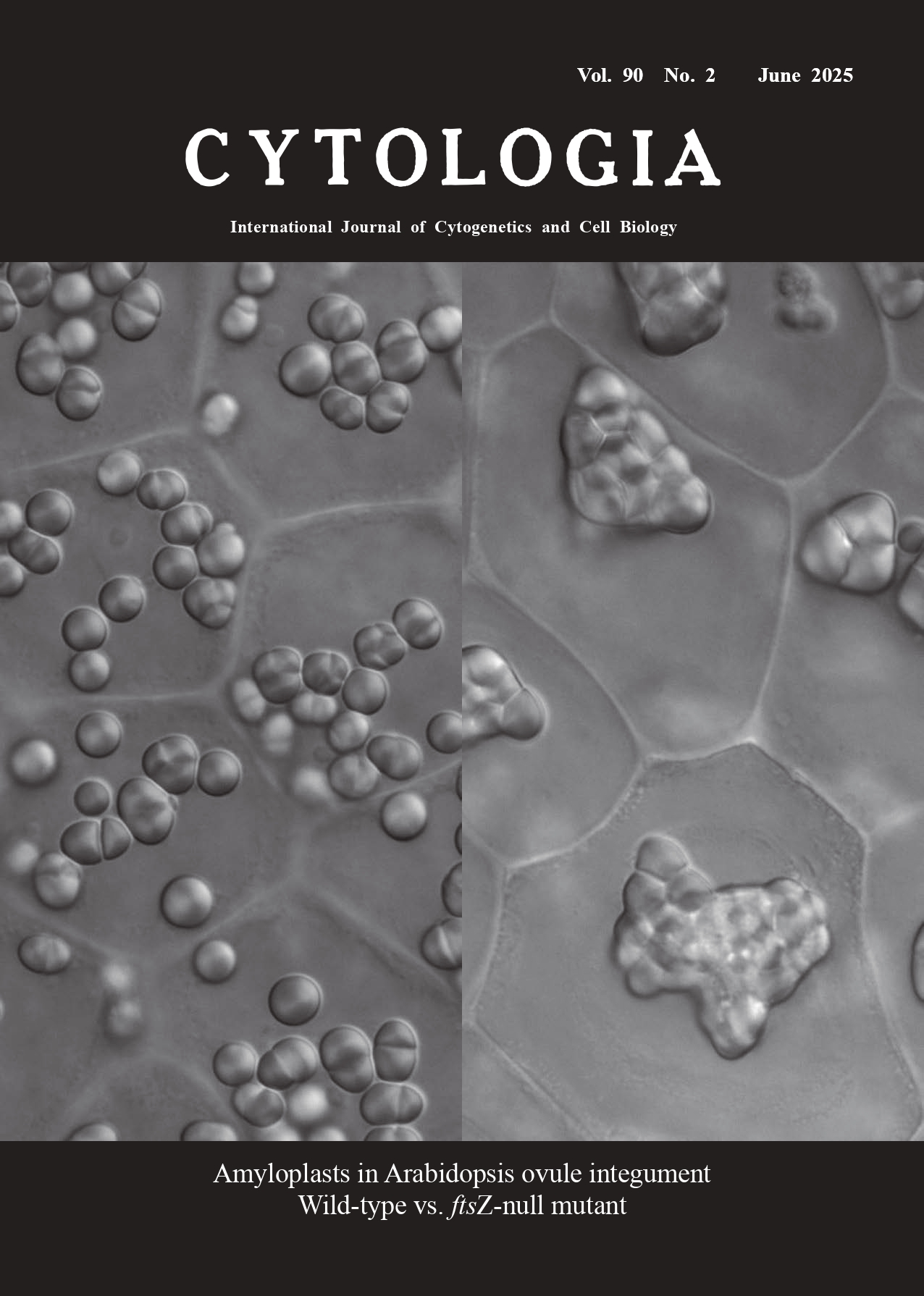 |
||
|---|---|---|---|
| Vol. 90 No.2 June 2025 | |||
| Technical Note | |||
|
|
|||
| Amyloplast imaging system in Arabidopsis ovule integument
Makoto T. Fujiwara1* and Ryuuichi D. Itoh2 1 Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, Tokyo 102–8554, Japan 2 Department of Chemistry, Biology and Marine Science, Faculty of Science, University of the Ryukyus, Senbaru 1, Nishihara, Okinawa 903–0213, Japan
Our imaging technique revealed distinct amyloplast morphologies in the ovule integument of wild-type and ftsZ-null mutants of A. thaliana. Wild-type cells contained round to ellipsoidal amyloplasts of relatively homogeneous size. By contrast, ftsZ-null mutant amyloplasts displayed severe morphological anomalies, characterized by irregular, enlarged amyloplasts with excessive stromule formation (Fujiwara et al. 2024). The number of amyloplasts per cell was low (1–3 in Phase III) in the ftsZ-null mutant compared to the wild type (9–15 amyloplasts in Phase III). The mutant amyloplasts also exhibited aberrant starch granule distribution, as starch granules were often localized within stromules in mutant amyloplasts but occupied most of the organelle volume in wild-type amyloplasts. These findings underscored the critical role of FtsZ in mediating division and maintaining structural integrity of amyloplasts. The absence of FtsZ disrupted envelope fission and led to unregulated amyloplast enlargement, while also impairing starch compartmentalization, highlighting its indispensability for normal amyloplast biogenesis and replication in A. thaliana. We hope that the system for imaging amyloplasts in A. thaliana ovule integument introduced here will serve as a foundation for future advances in our understanding of amyloplast biogenesis.
Fujiwara, M. T., Yoshioka, Y., Kazama, Y., Hirano, T., Niwa, Y., Moriyama, T., Sato, N., Abe, T., Yoshida, S., and Itoh, R. D. 2024. Principles of amyloplast replication in the ovule integuments of Arabidopsis thaliana. Plant Physiol. 196: 137–152. Schmitz, A. J., Glynn, J. M., Olson, B. J. S. C., Stokes, K. D., and Osteryoung, K. W. 2009. Arabidopsis FtsZ2-1 and FtsZ2-2 are functionally redundant, but FtsZ-based plastid division is not essential for chloroplast partitioning or plant growth and development. Mol. Plant 2: 1211–1222. * Corresponding author, e-mail: mtf1@mac.com DOI: 10.1508/cytologia.90.77 |
|||