| ON THE COVER | 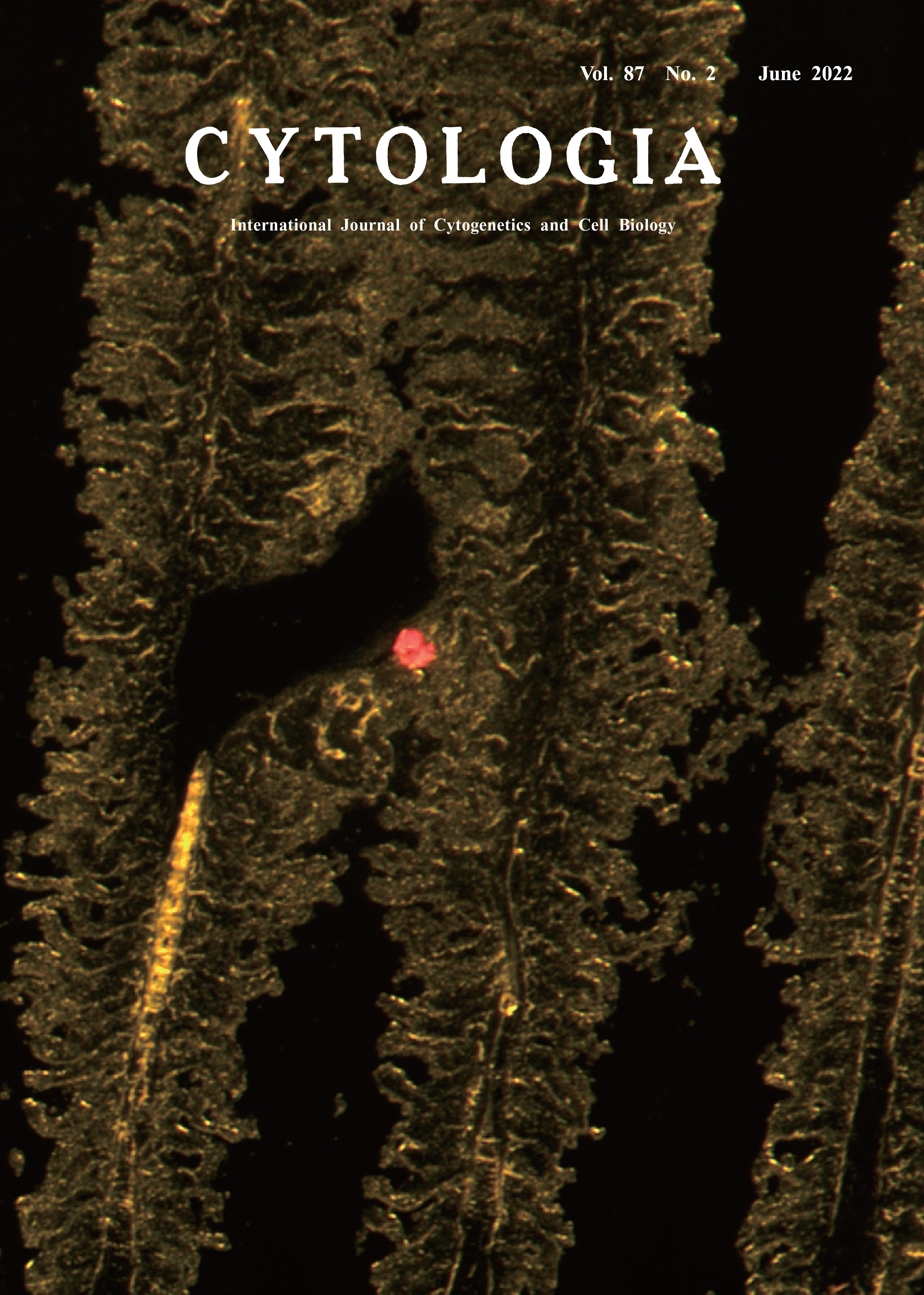 |
||
|---|---|---|---|
| Vol. 87 No.2 June 2022 | |||
| Technical Note | |||
|
|
|||
A Frozen-Section Procedure for Detecting Red-Tide Algae on the Gills of Aquaculture Fish Takafumi Katsumura1*, Toshiyuki Nishimaki1, Kogen Okita2, Keita Ishii2,Toshinori Takashi2, Toshifumi Yamatogi3, Motoyuki Ogawa1 and Tomoyuki Shikata4* 1 Department of Anatomy, Kitasato University School of Medicine, 1–15–1 Kitazato, Sagamihara, Kanagawa 252–0374, Japan 2 Fisheries Technology Institute, Japan Fisheries Research and Education Agency, 1551–8 Taira–machi, Nagasaki, Nagasaki 851–2213, Japan 3 Nagasaki Prefectural Institute of Fisheries, 1551–4 Taira, Nagasaki, Nagasaki 851–2213, Japan 4 Fisheries Technology Institute, Japan Fisheries Research and Education Agency, 122–7 Nunoura, Tamanoura–cho, Goto,Nagasaki 853–0508, Japan
Due to tremendous overgrowth of toxic algae, red tides cause significant economic losses in the aquaculture industry. Despite extensive studies, the precise mechanism through which red tides kill fish remains unclear (Matsuyama and Oda 2020). Histologic damage including congestion and edema has been observed in the gills of fish exposed to red-tide algae. However, algal cells in the gills have been demonstrated in surprisingly few cases. This infrequency may result from the inability of tissue fixation solutions to adhere red-tide algae to the gills, or the algal cells may be easily dislodged during the manipulation and chemical exposures involved with the preparation of sections. To overcome these problems, we applied a frozen-section method to the gills of an aquaculture fish exposed to a red-tide alga and prepared serial sections. Because no fixative or organic solvent is used, we presumed that this method would preserve the structures of the gill tissue and the algae itself. The cover shows a fluorescent image of a noxious redtide dinoflagellate, Karenia mikimotoi (Li et al. 2019), attached to the gill tissue of a bluefin tuna (Thunnus orientalis) larva that we prepared by using our frozen-section method. To obtain this image, we first cultured K. mikimotoi: IMR4 strain (isolated from Imari Bay, Japan) in ESM medium at ~180 μmol photons m-2 s-1, 22°C, 14 hL : 10 hD. We then exposed five tunas (average weight, 3.2 g; 48 days post-hatching) produced through artificial seedling to 16 L of K. mikimotoi culture (11,000 cells mL-1) in a round, 30-L, transparent, open-top aquaculture tank that was placed in a reinforced plastic fish tank containing temperature- controlled water adjusted to 22°C. The dissolved oxygen concentration was maintained at 6–7 mg L-1 by using an air pump. The gills were removed from a moribund fish after 69 min of the algae exposure and immediately placed in an embedding dish (Sakura Finetek Japan, Inc.), and OCT Compound (Sakura Finetek Japan, Inc.) was added as an embedding reagent to immobilize the tissue. To freeze the tissue, the embedding dish was placed for 90 s in a 500-mL beaker containing 300 mL isopentane (Nakalai Tesque Co., Ltd.) that had been cooled sufficiently by adding a small amount of dry ice. Frozen tissues were wrapped in aluminum foil and stored at -80°C until use. For sectioning, the frozen tissue block was transferred to a cryostat, CM1850 (Leica Microsystems, Inc.) and equilibrated at -20°C for 30 min to prevent the tissue block from cracking. The blocks were serially thin-sectioned at 10 μm. Sections were stretched on glass slides, and dried under a fan for 20 min to promote adhesion of the sections to the slides. The dried sections were observed for autofluorescence of algal chlorophyll by using an ET GFP LP filter (480/40 510LP M, Leica Microsystems, Inc.) and a fluorescence stereomicroscope, M205FCA TL3000-FC (Leica Microsystems, Inc.). The images were acquired by using a digital camera, DFC7000T-DT (Leica Microsystems, Inc.). The cover photo shows the adhesion of K. mikimotoi cells (red fluorescence) between the secondary lamellae (yellow fluorescence) of bluefin tuna. The gill lamellae have not retained their structure, indicating that K. mikimotoi has damaged them.
Li, X., Yan, T., Yu, R. and Zhou, M. 2019. A review of Karenia mikimotoi: Bloom events, physiology, toxicity and toxic mechanism. Harmful Algae 90: 101702. Matsuyama, Y. and Oda, T. 2020. Toxic Effects of Harmful Algal Blooms on Finfish and Shellfish. In: Konur, O. (ed.). Handbook of Algal Science, Technology, and Medicine. Elsevier B.V., Amsterdam. pp. 543–560. * Corresponding author, e-mail: tk@med.kitasato-u.ac.jp; shikatat@affrc.go.jp DOI: 10.1508/cytologia.87.67 |
|||