| ON THE COVER | 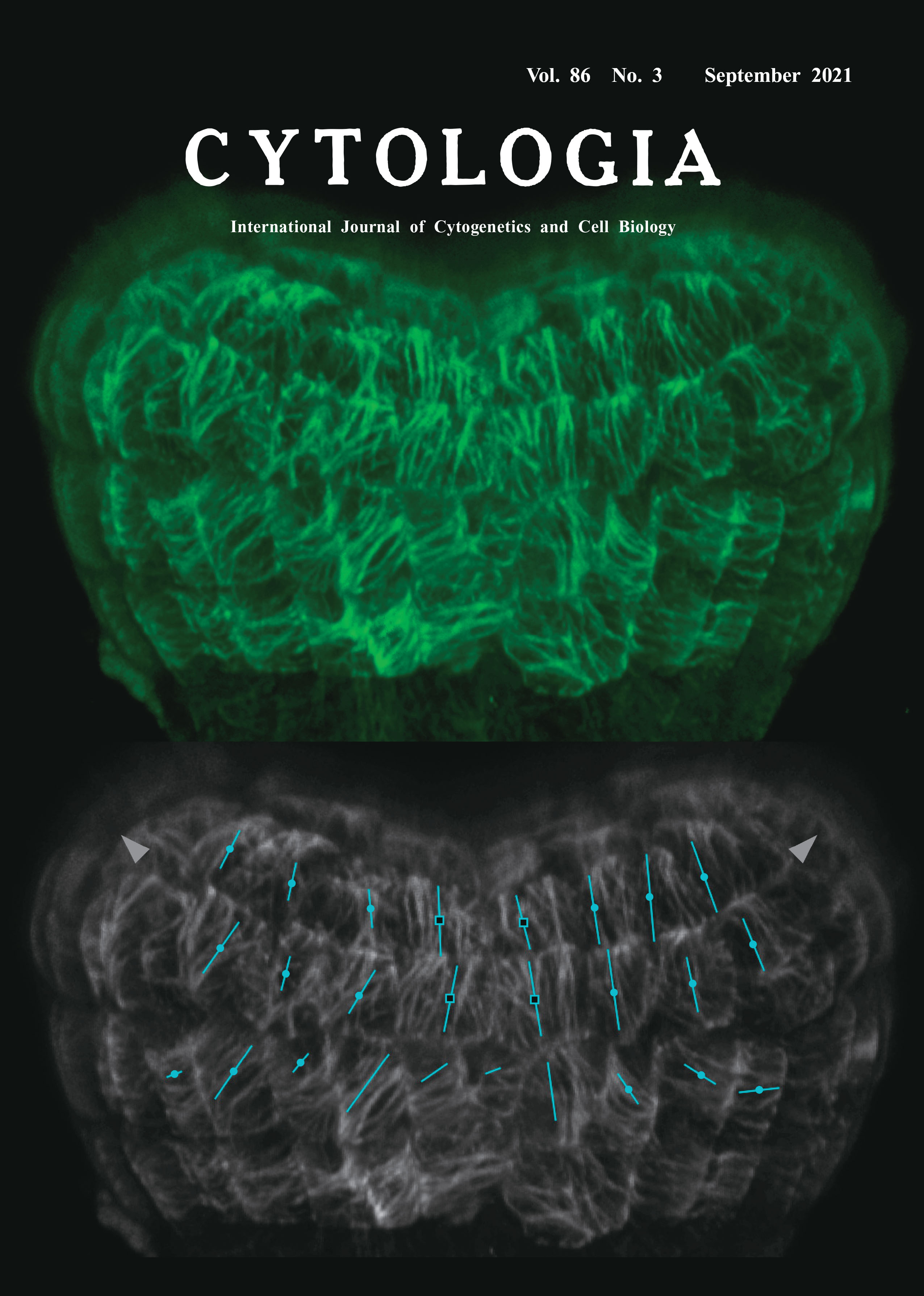 |
||
|---|---|---|---|
| Vol. 86 No.3 September 2021 | |||
| Technical Note | |||
|
|
|||
Visualization and Quantification of Cortical Microtubules in the Apical Region of the Arabidopsis thaliana Embryo Ayana Takahama1, and Mitsuhiro Aida2* 1 Faculty of Science, Kumamoto University, 2–39–1 Kurokami, Chuo-ku, Kumamoto 860–8555, Japan 2 International Research Organization for Advanced Science and Technology (IROAST), Kumamoto University, 2–39–1 Kurokami, Chuo-ku, Kumamoto 860–8555, Japan
Morphogenesis in plants is highly dependent on the coordinated patterns of cell division and elongation. Cortical microtubules (CMTs) have important roles in controlling cell elongation patterns through the regulation of oriented deposition of cellulose microfibrils, a main determinant of anisotropic cellular growth (Landrein and Hamant 2013). At the multicellular level, the orientation of CMTs is coordinately patterned across the tissue surface and affects directional growth. A reliable method to visualize and quantify the patterns of CMTs is thus important for understand mechanisms underlying growth regulation of plant organs. In dicotyledonous plants, a pair of cotyledon primordia are initiated at symmetric positions in the apical region of the embryo. This morphogenetic process is associated with anisotropic growth of the primordia and growth suppression in their boundary region. To visualize the patterns of CMTs during cotyledon initiation in the dicot Arabidopsis thaliana, we used a reporter line expressing a GFP-TUB6 fusion protein (Fujita et al. 2013). Because GFP-labelled microtubules are unstable when embryos are excised from developing seeds, we preincubated seeds for 30 min in a buffer containing Taxol and then extracted embryos in the same buffer prior to observation, a method that has been successfully applied to proembryos (Liao and Weijers 2018). We then collected a stack of 163 serial optical sections of an early-heart-stage embryo with the 0.3 μm intervals and the image size 512×512, using the UPlanSApo 60× objective lens (NA=1.35) equipped on the confocal microscope FV3000 (Olympus, Tokyo). The collected images were then subjected to 3D reconstruction using the ImageJ plugin VolumeViewer (https://imagej.net/ plugins/volume-viewer.html) with the Mode and Interpolation parameters set to Volume and Tricubic sharp, respectively, resulting in the visualization of CMTs that lay just beneath the embryo surface (top panel, green). The reconstructed image was then subjected to the quantitative analysis using the FibrilTool plugin (Boudaoud et al. 2014). In the bottom panel, the same image as that depicted in the top panel is shown in grayscale, overlaid by lines with various orientation and length, each representing the average angle and anisotropy of CMTs in a given cell. The approximate growth directions of the cotyledon primordia are indicated by arrowheads. The results show that the CMTs in the primordia boundary region (open squares) tended to align vertically with relatively high anisotropy whereas those in the primordia (closed circles) were laid so that they encircle the growth axis, reflecting the regional differences in their growth property.
Boudaoud, A., Burian, A., Borowska-Wykręt, D., Uyttewaal, M., Wrzalik, R., Kwiatkowska, D. and Hamant, O. 2014. FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat. Protoc. 9: 457–463. Fujita, S., Pytela, J., Hotta, T., Kato, T., Hamada, T., Akamatsu, R., Ishida, Y., Kutsuna, N., Hasezawa, S., Nomura, Y., Nakagami, H. and Hashimoto, T. 2013. An atypical tubulin kinase mediates stress-induced microtubule depolymerization in Arabidopsis. Curr. Biol. 23: 1969–1978. Landrein, B. and Hamant, O. 2013. How mechanical stress controls microtubule behavior and morphogenesis in plants: History, experiments and revisited theories. Plant J. 75: 324–338. Liao, C. Y. and Weijers, D. 2018. A toolkit for studying cellular reorganization during early embryogenesis in Arabidopsis thaliana. Plant J. 93: 963–976. * Corresponding author, e-mail: m-aida@kumamoto-u.ac.jp DOI: 10.1508/cytologia.86.181 | |||