| ON THE COVER | 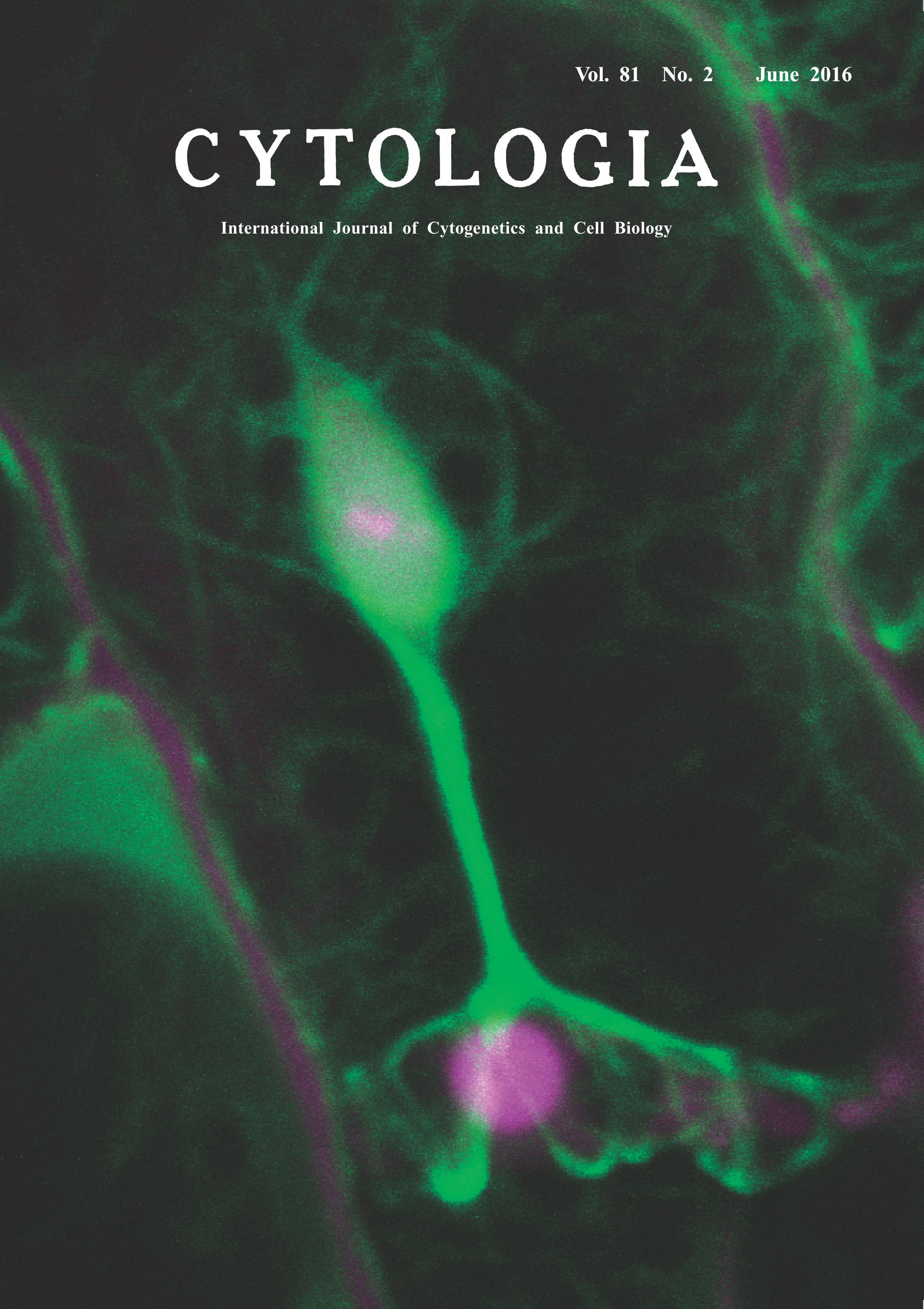 |
|
|---|---|---|
| Vol. 81 No.2 June 2016 | ||
| Technical note | ||
|
|
||
| Visualization of Host Actin Microfilament Dynamicity
upon Obligate Biotrophic Pathogen Infection Noriko Inada* Graduate School of Biological Sciences, Nara Institute of Science and Technology, Takayama-cho, Ikoma, Nara 630–0192, Japan Received December 8, 2015; accepted May 12, 2016 Obligate biotrophic fungal pathogens infect living host cells by manipulating host cellular functions. The molecular mechanism of the host plant–obligate biotroph interaction remains unknown. We have established a live-imaging method to investigate the dynamicity of the host-pathogen interaction using host plant Arabidopsis thaliana and A. thaliana-adapted powdery mildew fungus Golovinomyces orontii. Powdery mildew affects nearly 10000 plants, and thus is one of the most important plant diseases. Powdery mildew fungi only infect host epidermal cells, and thus their infection processes are easily analyzed with a microscope. Using the above pathosystem, we recently observed the dynamicity of host actin microfilaments (AFs) labeled with GFP-hTalin and found that powdery mildew fungal haustorium, a specialized infection organ formed in the host cell, is surrounded by host AFs. The movement of AFs around the haustorium is somewhat slower than that of AFs at the cell periphery. In addition, the density of AFs around the haustorium increase as the haustorium becomes mature (Inada et al. 2016a). As intact host AFs are required for powdery mildew infection (Inada et al. 2016b), haustorium-surrounding AFs may contribute to infection as a result of fungal manipulation. Here I show AFs around the haustorium connected to those around the host nucleus. First, 2 mm diameter discs were cut from mature leaves expressing GFP-hTalin at one day post inoculation (dpi) of G. orontii using a cork borer, and incubated with 0.5% propidium iodide (PI) in 2.5% mannitol and 0.01% Silwet solution to stain fungal structures. The discs were then rinsed and mounted with 2.5% mannitol and 0.01% Silwet solution, covered with a slice of 1% agarose to avoid dehydration and movement of tissue, and subjected to observation with a confocal scanning laser microscope FV1000 (Olympus, Tokyo, Japan) equipped with a ×40 objective lens (N.A. 0.8, Olympus). Both GFP and PI were excited with a 488-nm argon laser, and the fluorescence was collected through bandpasses of 500–530 nm for GFP and 555–655 nm for PI. Z-Stack images were obtained with a zoom factor of 6 at 1μm step size and were processed with ImageJ software version 1.48v (National Institute of Health, Bethesda, MD, U.S.A.). At 1 dpi, the host nucleus is observed to be closely attached to the haustorium in nearly 50% of infected cells. This image, showing a haustorium (magenta, located at the lower half of the image) connected with the host nucleus (stained also with PI, magenda, and labeled with GFP-hTalin, green, located at the upper half of the image) with host AFs (green), suggests an importance of host AFs in this event. As the host nuclear factor also contributes to the susceptibility (Inada et al. 2016b), this nuclear relocalization could also be a result of fungal manipulation. Our live-imaging method will greatly contribute to revealing the mechanism of this intricate interaction.
Inada, N., Higaki, T. and Hasezawa, S. 2016b. Nuclear function of subclass I actin-depolymerizing factor contributes to susceptibility in Arabidopsis to an adapted powdery mildew fungus. Plant Physiol. 170: 1420–1434. *Corresponding author, e-mail:norikoi@bs.naist.jp DOI: 10.1508/cytologia.81.119 |
||